| |
|---|
|
品名: | Pierce Chromogenic Endotoxin Quant Kit |
产品简介:
规格
| Assay: |
End-point Chromogenic |
| 测定范围: |
0.01-0.1 EU/mL, 0.1-1.0 EU/mL |
| Assay Sensitivity: |
0.01 EU/ml |
| Detection Method: |
Colorimetric |
| Product Line: |
Pierce? |
| Shipping Condition: |
Wet Ice |
| 足够用于: |
60 microplate-well assays containing 0.01 to 1 endotoxin units/mL |
内容及储存
* Lyophilized amebocyte lysate
* E.coli endotoxin standard
* Chromgenic substrate
* Endotoxin-free water
Store at 2–8°C upon receipt.
描述
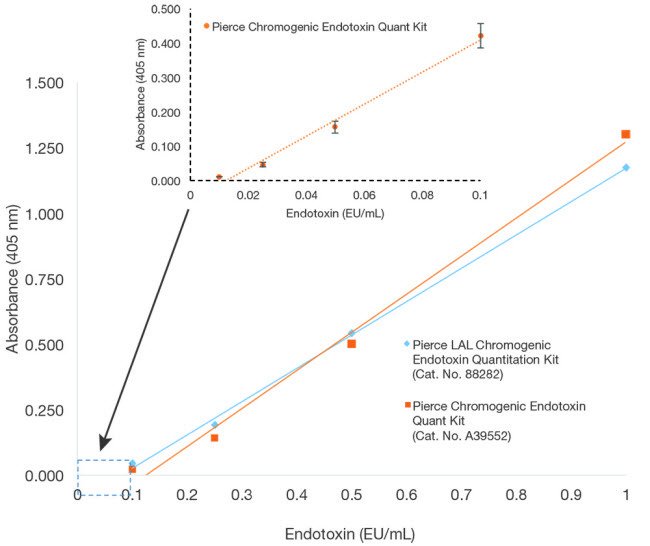
The Thermo Scientific Pierce Chromogenic Endotoxin Quant Kit is a highly sensitive endpoint assay that accurately measures and detects endotoxin (lipopolysaccharide) in a protein, peptide, nucleic acid, or antibody sample using the amebocyte lysate assay. The kit enables detection within two linear sensitivity ranges of 0.01–0.1 EU/mL and 0.1–1.0 EU/mL.
Features and benefits of the Pierce Chromogenic Endotoxin Quant Kit include:
*
Highly sensitive with a broad range—detect as little as 0.01 EU/mL to 1 EU/mL
*
Specific—no interference from ?-glucans and suitable for wide range of samples, including protein, vaccine, plasmid, DNA, RNA
*
Fast—perform assay in as little as 20 minutes
*
End-point chromogenic assay—measure with a standard spectrophotometer or plate reader at 405–410 nm
The Pierce Chromogenic Endotoxin Quant Kit is an end-point chromogenic endotoxin detection assay based on the amebocyte lysate method, which measures endotoxin through the interaction of the endotoxin with the proenzyme Factor C found in circulating amebocytes of the horseshoe crab. The proteolytic activity of this proenzyme is activated in the presence of lipopolysaccharides (endotoxins) derived from the outer cell membrane of gram-negative bacteria such as E.coli. Endotoxin levels are determined by measuring the activity of Factor C in the presence of a synthetic peptide substrate that releases p-nitroaniline (pNA) after proteolysis, producing a yellow color that can be measured at an absorbance of 405 nm.
Endotoxin levels in the samples are accurately determined using the included endotoxin standard of known concentration that is derived from E.coli strain O111: B4. Determining endotoxin levels is important to assess the efficiency of endotoxin removal methods and prevent endotoxic shock, inflammation, and/or sepsis in tissue culture cells and animals injected with endotoxin-contaminated proteins.
Applications: quantitation of endotoxin levels in a protein, peptide, antibody, or nucleic acid samples
Related products:Pierce High Capacity Endotoxin Removal ResinPierce High Capacity Endotoxin Removal Spin Columns
For Research Use Only. Not for use in diagnostic procedures.
For Research Use Only. Not for use in diagnostic procedures.